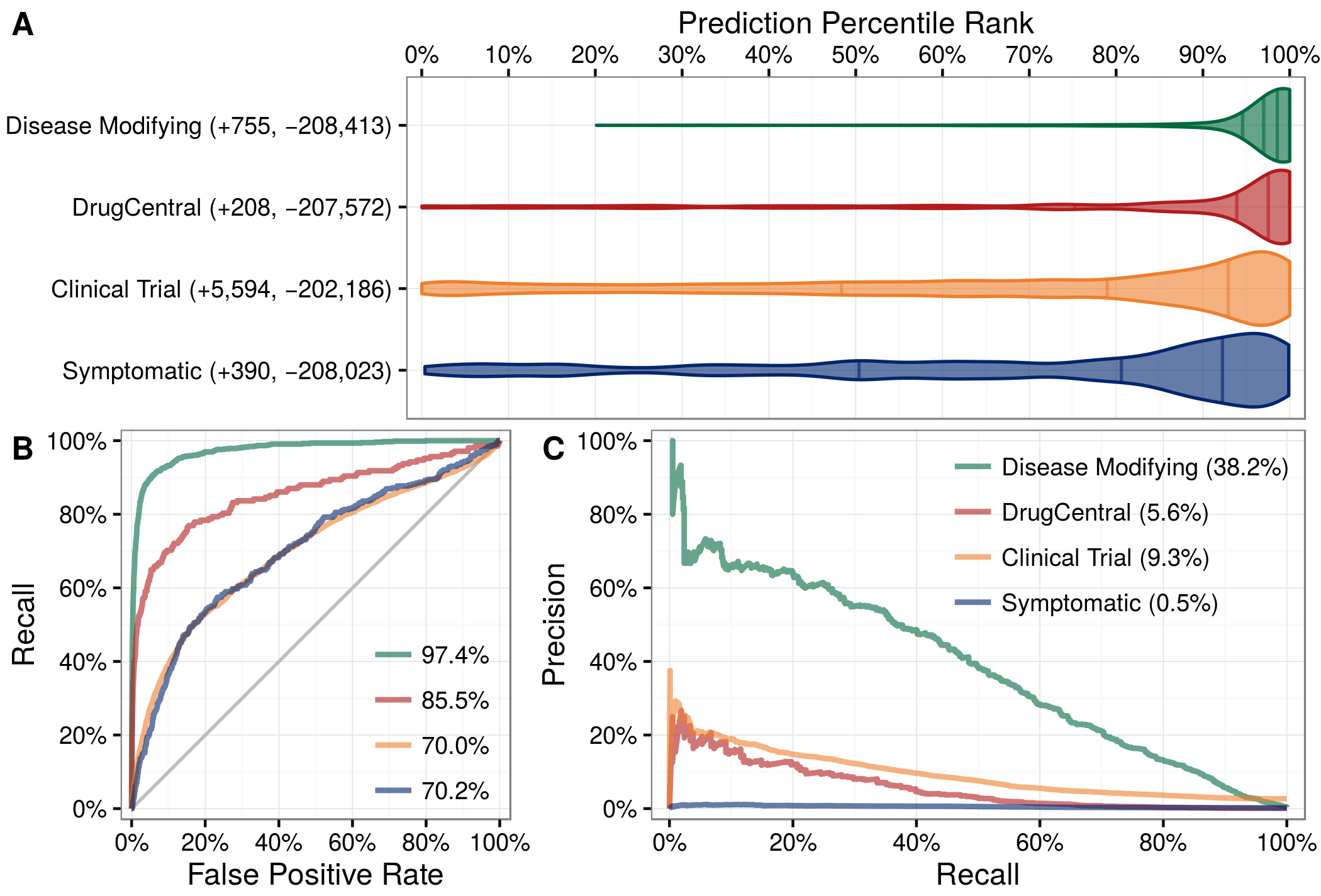
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | Incorporating DrugCentral data in our networkDaniel Himmelstein, Oleg Ursu, Mike Gilson, Pouya Khankhanian, Tudor Oprea (2016) Thinklab. doi: 10.15363/thinklab.d186 |
| 8. | |
| 9. | |
| 10. | |
| 11. | Unifying disease vocabulariesDaniel Himmelstein, Tong Shu Li (2015) Thinklab. doi: 10.15363/thinklab.d44 |
| 12. | Disease Ontology 2015 update: an expanded and updated database of human diseases for linking biomedical knowledge through disease dataW. A. Kibbe, C. Arze, V. Felix, E. Mitraka, E. Bolton, G. Fu, C. J. Mungall, J. X. Binder, J. Malone, D. Vasant, H. Parkinson, L. M. Schriml (2014) Nucleic Acids Research. doi: 10.1093/nar/gku1011 |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | Citation not found. |
| 18. | 15 Years of Clinical Experience With Bupropion HClMaurizio Fava, A. John Rush, Michael E. Thase, Anita Clayton, Stephen M. Stahl, James F. Pradko, J. Andrew Johnston (2005) Prim. Care Companion J. Clin. Psychiatry. doi: 10.4088/pcc.v07n0305 |
| 19. | Bupropion for Smoking Cessation: A ReviewElisa K. Tong, Timothy P. Carmody, Joel A. Simon (2006) COMP. doi: 10.1385/comp:32:1:26 |
| 20. | A Comparison of Sustained-Release Bupropion and Placebo for Smoking CessationRichard D. Hurt, David P.L. Sachs, Elbert D. Glover, Kenneth P. Offord, J. Andrew Johnston, Lowell C. Dale, Moise A. Khayrallah, Darrell R. Schroeder, Penny N. Glover, C. Rollynn Sullivan, Ivana T. Croghan, Pamela M. Sullivan (1997) New England Journal of Medicine. doi: 10.1056/nejm199710233371703 |
| 21. | |
| 22. | |
| 23. | Efficacy and Safety of Varenicline for Smoking CessationJ. Taylor Hays, Jon O. Ebbert, Amit Sood (2008) The American Journal of Medicine. doi: 10.1016/j.amjmed.2008.01.017 |
| 24. | Placebo-Controlled Trial of Cytisine for Smoking CessationRobert West, Witold Zatonski, Magdalena Cedzynska, Dorota Lewandowska, Joanna Pazik, Paul Aveyard, John Stapleton (2011) New England Journal of Medicine. doi: 10.1056/NEJMoa1102035 |
| 25. | Cytisine versus Nicotine for Smoking CessationNatalie Walker, Colin Howe, Marewa Glover, Hayden McRobbie, Joanne Barnes, Vili Nosa, Varsha Parag, Bruce Bassett, Christopher Bullen (2014) New England Journal of Medicine. doi: 10.1056/NEJMoa1407764 |
| 26. | Nicotine receptor partial agonists for smoking cessationKate Cahill, Nicola Lindson-Hawley, Kyla H Thomas, Thomas R Fanshawe, Tim Lancaster (2016) Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.CD006103.pub7 |
| 27. | |
| 28. | |
| 29. | Understanding multicellular function and disease with human tissue-specific networksCasey S Greene, Arjun Krishnan, Aaron K Wong, Emanuela Ricciotti, Rene A Zelaya, Daniel S Himmelstein, Ran Zhang, Boris M Hartmann, Elena Zaslavsky, Stuart C Sealfon, Daniel I Chasman, Garret A FitzGerald, Kara Dolinski, Tilo Grosser, Olga G Troyanskaya (2015) Nat Genet. doi: 10.1038/ng.3259 |
| 30. | A Placebo-Controlled Trial of Oral Cladribine for Relapsing Multiple SclerosisGavin Giovannoni, Giancarlo Comi, Stuart Cook, Kottil Rammohan, Peter Rieckmann, Per Soelberg Sørensen, Patrick Vermersch, Peter Chang, Anthony Hamlett, Bruno Musch, Steven J. Greenberg (2010) New England Journal of Medicine. doi: 10.1056/NEJMoa0902533 |
| 31. | |
| 32. | CladribineThomas P. Leist, Robert Weissert (2011) Clinical Neuropharmacology. doi: 10.1097/WNF.0b013e318204cd90 |
| 33. | No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribineJulia Pakpoor, Giulio Disanto, Daniel R. Altmann, Sue Pavitt, Benjamin P. Turner, Monica Marta, Gunnar Juliusson, David Baker, Jeremy Chataway, Klaus Schmierer (2015) Neurology - Neuroimmunology Neuroinflammation. doi: 10.1212/nxi.0000000000000158 |
| 34. | Azathioprine for multiple sclerosisIlaria Casetta, Gerardo Iuliano, Graziella Filippini (2007) Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.CD003982.pub2 |
| 35. | Azathioprine versus Beta Interferons for Relapsing-Remitting Multiple Sclerosis: A Multicentre Randomized Non-Inferiority TrialLuca Massacesi, Irene Tramacere, Salvatore Amoroso, Mario A. Battaglia, Maria Donata Benedetti, Graziella Filippini, Loredana La Mantia, Anna Repice, Alessandra Solari, Gioacchino Tedeschi, Clara Milanese (2014) PLoS ONE. doi: 10.1371/journal.pone.0113371 |
| 36. | |
| 37. | Standing the test of time: targeting thymidylate biosynthesis in cancer therapyPeter M. Wilson, Peter V. Danenberg, Patrick G. Johnston, Heinz-Josef Lenz, Robert D. Ladner (2014) Nature Reviews Clinical Oncology. doi: 10.1038/nrclinonc.2014.51 |
| 38. | Randomized Trial of TAS-102 for Refractory Metastatic Colorectal CancerRobert J. Mayer, Eric Van Cutsem, Alfredo Falcone, Takayuki Yoshino, Rocio Garcia-Carbonero, Nobuyuki Mizunuma, Kentaro Yamazaki, Yasuhiro Shimada, Josep Tabernero, Yoshito Komatsu, Alberto Sobrero, Eveline Boucher, Marc Peeters, Ben Tran, Heinz-Josef Lenz, Alberto Zaniboni, Howard Hochster, James M. Cleary, Hans Prenen, Fabio Benedetti, Hirokazu Mizuguchi, Lukas Makris, Masanobu Ito, Atsushi Ohtsu (2015) New England Journal of Medicine. doi: 10.1056/NEJMoa1414325 |
| 39. | |
| 40. | Review on TAS-102 development and its use for metastatic colorectal cancerJose Mauricio Mota, Leonardo G. Fonseca, Maria Ignez Braghiroli, Paulo M. Hoff (2016) Critical Reviews in Oncology/Hematology. doi: 10.1016/j.critrevonc.2016.05.015 |
| 41. | |
| 42. | |
| 43. | Methotrexate for multiple sclerosisOrla Gray, Gavin V McDonnell, Raeburn B Forbes (2004) Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.CD003208.pub2 |
| 44. | Chemotherapeutics in the treatment of multiple sclerosisB. C. Kieseier, D. R. Jeffery (2010) Therapeutic Advances in Neurological Disorders. doi: 10.1177/1756285610379885 |
| 45. | New avenues for anti-epileptic drug discovery and developmentWolfgang Löscher, Henrik Klitgaard, Roy E. Twyman, Dieter Schmidt (2013) Nature Reviews Drug Discovery. doi: 10.1038/nrd4126 |
| 46. | |
| 47. | Seizure Activity and Off-Label Use of TiagabineCharlene M. Flowers, Judith A. Racoosin, Cindy Kortepeter (2006) New England Journal of Medicine. doi: 10.1056/NEJMc055301 |
| 48. | |
| 49. | |
| 50. | Acute amitriptyline intoxication: an analysis of 44 childrenHüseyin Çaksen, Sinan Akbayram, Dursun Odabaş, Hanefi Özbek, Mehmet Erol, Cihangir Akgün, Oğuz Tuncer, Cahide Yılmaz (2006) Human & Experimental Toxicology. doi: 10.1191/0960327106ht511oa |
| 51. | |
| 52. | Sevoflurane and epileptiform EEG changesISABELLE CONSTANT, ROBERT SEEMAN, ISABELLE MURAT (2005) Pediatric Anesthesia. doi: 10.1111/j.1460-9592.2004.01538.x |
| 53. | |
| 54. | Anaesthesia and epilepsyA. Perks, S. Cheema, R. Mohanraj (2012) British Journal of Anaesthesia. doi: 10.1093/bja/aes027 |
| 55. | |
| 56. | Discovery and development of clofarabine: a nucleoside analogue for treating cancerPeter L. Bonate, Larry Arthaud, William R. Cantrell, Katherine Stephenson, John A. Secrist, Steve Weitman (2006) Nature Reviews Drug Discovery. doi: 10.1038/nrd2055 |
| 57. | |
| 58. | Mechanisms of anti-cancer action and pharmacology of clofarabineAnna Zhenchuk, Koroush Lotfi, Gunnar Juliusson, Freidoun Albertioni (2009) Biochemical Pharmacology. doi: 10.1016/j.bcp.2009.06.094 |
| 59. | |
| 60. | A Placebo-Controlled Trial of Oral Cladribine for Relapsing Multiple SclerosisGavin Giovannoni, Giancarlo Comi, Stuart Cook, Kottil Rammohan, Peter Rieckmann, Per Soelberg Sørensen, Patrick Vermersch, Peter Chang, Anthony Hamlett, Bruno Musch, Steven J. Greenberg (2010) New England Journal of Medicine. doi: 10.1056/NEJMoa0902533 |
| 61. | |
| 62. | Fludarabine add-on therapy in interferon-beta-treated patients with multiple sclerosis experiencing breakthrough diseaseS. J. Greenberg, R. Zivadinov, P. Lee-Kwen, J. Sharma, M. Planter, M. Umhauer, N. Glenister, R. Bakshi (2016) Therapeutic Advances in Neurological Disorders. doi: 10.1177/1756285615626049 |
| 63. | Azathioprine versus Beta Interferons for Relapsing-Remitting Multiple Sclerosis: A Multicentre Randomized Non-Inferiority TrialLuca Massacesi, Irene Tramacere, Salvatore Amoroso, Mario A. Battaglia, Maria Donata Benedetti, Graziella Filippini, Loredana La Mantia, Anna Repice, Alessandra Solari, Gioacchino Tedeschi, Clara Milanese (2014) PLoS ONE. doi: 10.1371/journal.pone.0113371 |
| 64. | |
| 65. | |
| 66. | Prediction in epilepsyPouya Khankhanian, Daniel Himmelstein (2016) Thinklab. doi: 10.15363/thinklab.d224 |
| 67. | Discovery and development of clofarabine: a nucleoside analogue for treating cancerPeter L. Bonate, Larry Arthaud, William R. Cantrell, Katherine Stephenson, John A. Secrist, Steve Weitman (2006) Nature Reviews Drug Discovery. doi: 10.1038/nrd2055 |
| 68. | Chemotherapeutics in the treatment of multiple sclerosisB. C. Kieseier, D. R. Jeffery (2010) Therapeutic Advances in Neurological Disorders. doi: 10.1177/1756285610379885 |
| 69. | A Placebo-Controlled Trial of Oral Cladribine for Relapsing Multiple SclerosisGavin Giovannoni, Giancarlo Comi, Stuart Cook, Kottil Rammohan, Peter Rieckmann, Per Soelberg Sørensen, Patrick Vermersch, Peter Chang, Anthony Hamlett, Bruno Musch, Steven J. Greenberg (2010) New England Journal of Medicine. doi: 10.1056/NEJMoa0902533 |
| 70. | Azathioprine for multiple sclerosisIlaria Casetta, Gerardo Iuliano, Graziella Filippini (2007) Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.CD003982.pub2 |
| 71. | Mechanisms of anti-cancer action and pharmacology of clofarabineAnna Zhenchuk, Koroush Lotfi, Gunnar Juliusson, Freidoun Albertioni (2009) Biochemical Pharmacology. doi: 10.1016/j.bcp.2009.06.094 |