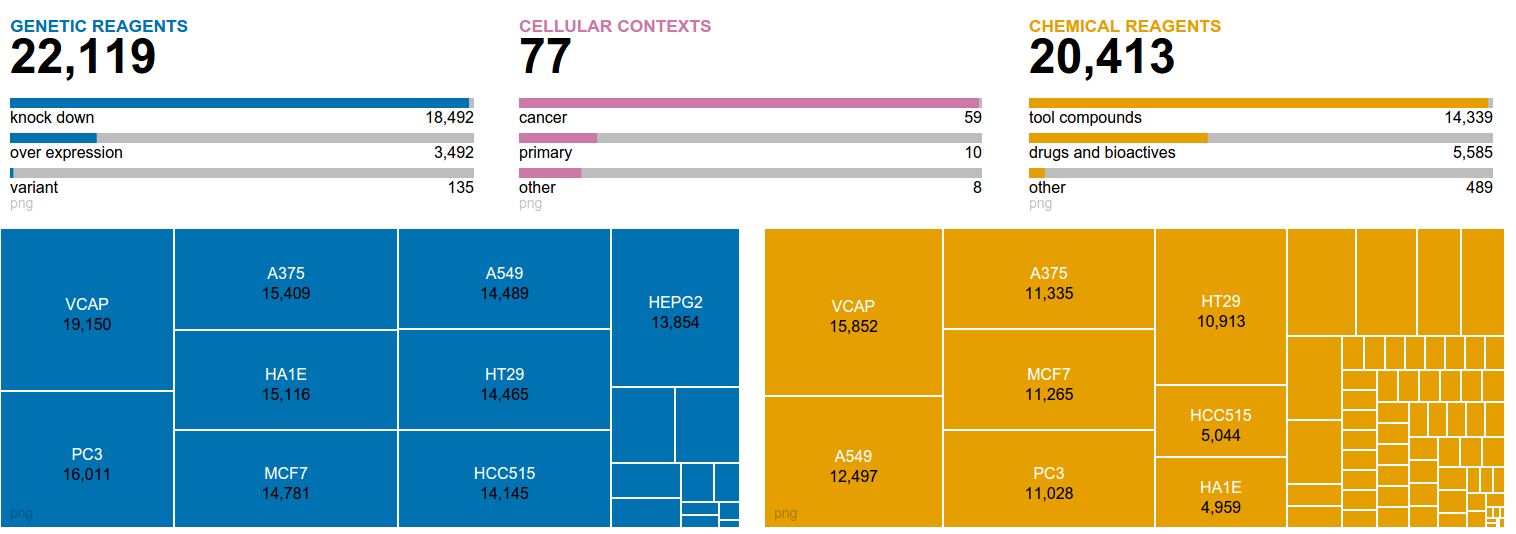
|
|
|
Status:
Open
Views
166
Topics
Referenced by
Cite this as
Daniel Himmelstein, Venkat Malladi, Alessandro Didonna, Alexey Strokach (2015) Suggestions for additional information types?. Thinklab. doi:10.15363/thinklab.d22
License
Share
|