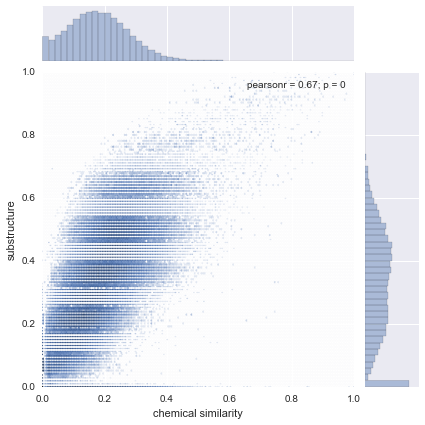
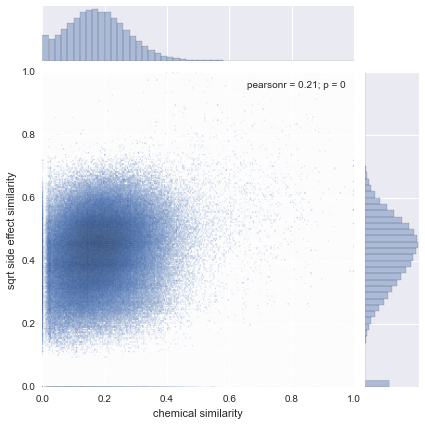
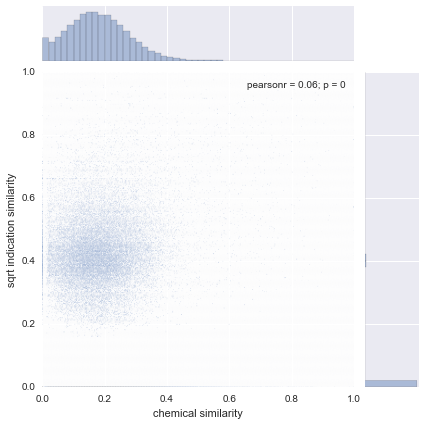
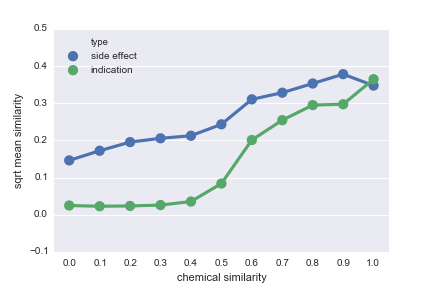
|
|
|
Status:
Completed
Views
178
Topics
Referenced by
Cite this as
Daniel Himmelstein, Leo Brueggeman, Sabrina Chen, Lars Juhl Jensen (2015) Assessing the quality and applicability of the SIDER 2 resource. Thinklab. doi:10.15363/thinklab.d30
License
Share
|